Organic Chemistry Laboratory II
Dehydration of Methylcyclohexanols: An
E1 Elimination
Experiment Description and Background
| Fractional distillation is used to separate liquids that are soluble in each other and boil within 25 °C of each other. The difference between a fractional distillation and a simple distillation is the use of a fractionating column (Vigreux or straight). The long column, filled with packing material helps to enhance the separation of the components of the mixture, ultimately enriching the distillate in the lower boiling component. In the case of this experiment, fractional distillation is used to separate the product cyclohexenes from the by-product, water. However, the fractional distillation does not provide a means to completely and thoroughly separate the two components. A residual amount of a cyclohexene-water mixture remains trapped on the packing material of the fractionating column. If the cyclohexene component of this mixture is not retrieved from the column, the yield of the reaction may be compromised. |
General Mechanism of the E1 Reaction
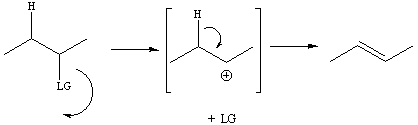
The first step of the E1 mechanism is the same as the
first
step of the SN1 reaction. The bond between the leaving
group
and the sp3 carbon bonded to the leaving group breaks
to
generate
a carbocation. An adjacent sp3 carbon then
gives
up electrons from one of its C-H bonds to form a new pi bond
between
the
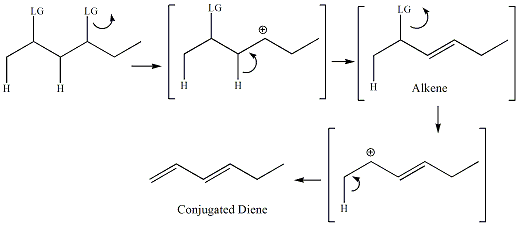
carbocation carbon and the adjacent carbon. For diene
formation,
the mechanism occurs so that one pi bond is completely formed and
then
the second pi bond is formed in the two step sequence.
| Alkenes
|
Dienes |
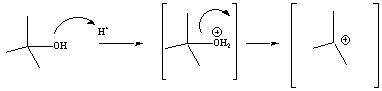
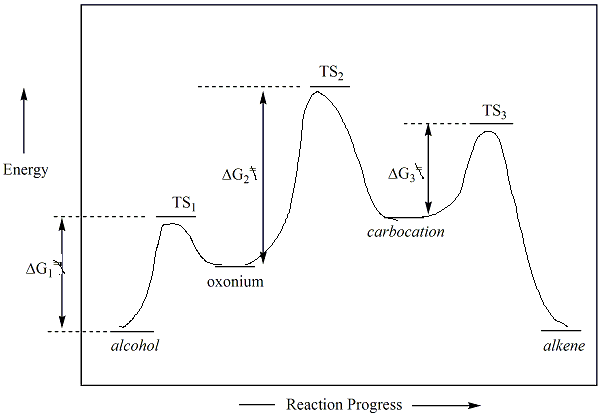
For alcohols, the OH group must first be activated with acid
before
it
will become a good leaving group (LG). This is frequently
done
using a strong acid (catalyst) with a conjugate base that is not
nucleophilic. Sulfuric and phosphoric acids are good
activators
for E1 reactions. The oxygen atom of the alcohol
becomes
protonated to
form an oxonuim ion. Water is then the leaving group.
Loss
of the water leaving group results in the formation of a
carbocation
which then undergoes the E1 elimination as
described
above. Hydrohalic acids (i.e., HX) are not
typically used as catalysts for elimination reactions because the
resulting halide
ion (conjugate base) may serve as a
nucleophile. As a result products of SN1 reactions
will be generated along with the E1 products.
In this experiment, cyclohexenes are prepared from cyclohexanols. Each of these compounds have unique functional groups and should therefore be easily distinguished from one another by IR spectroscopic analysis. Most characteristically, the starting material, methylcyclohexanol, will have a broad absorbance in the range between 3200- 3600 cm-1 corresponding to its alcohol O-H bond. This absorbance should be absent in the IR spectrum of the cyclohexene product. Some caution must be exercised however, since specific reaction conditions and by-products of the reaction must be taken into account before interpreting the IR spectra. Water is a major by-product of this reaction and as discussed above, may very likely contaminate the product, even after a fractional distillation is done. If water does in fact contaminate the product, an absorbance at 3200-3600cm-1 may appear in the IR spectrum of the product due to the O-H bond of water. Alternatively, an absorbance in this region in the IR spectrum of the product might also be interpreted as contamination by unreacted cyclohexanol. Consideration of the reaction procedure/conditions should allow you to rule this out. Why?
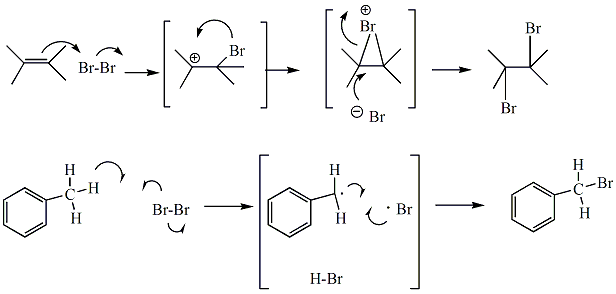
Reaction of bromine with alkenes or alkynes occurs through an electrophilic addition mechanism to generate dihaloalkanes. The brown colored bromine reagent becomes incorporated into the organic hydrocarbon, producing a colorless product. If the bromine reagent is used in excess, or if no reaction occurs, the solution will remain brownish in color. Carbon tetrachloride (CCl4) serves as solvent. Cyclohexenes, the products in the E1 elimination would be expected to react readily with Br2 in CCl4 to form the dihaloalkane. Bromine is a dark brown liquid, and even solutions of bromine (such as Br2 in CCl4) are brown, light brown or yellow, depending on the concentration. Dihaloalkanes, are colorless compounds. When Br2 in CCl4 reacts with an alkene, the colored bromine becomes incorporated into the colorless organic dihaloalkane, and the reaction solution goes from brown (or yellow) to colorless. Br2 in CCl4 does not react with alcohols, and the solution of Br2 in CCl4 would be expected to remain brown, light brown or yellow upon reaction with methylcyclohexanols. Bromine may also react with benzylic atoms through a radical halogenation mechanism. Even without a specific initiator, products from benzylic bromination are often detected in this test. In the reaction of bromine with toluene, the pi bonds of the benzene ring do not react, however, the benzylic atom of toluene will be brominated in this reaction.
Jones
Oxidation
Test
Jones reagent is a powerful oxidizing agent (CrO3/H2SO4)
that
is used primarily to convert primary alcohols to carboxylic acids,
and
secondary alcohols to ketones. Tertiary alcohols do not
react
with
Jones reagent. The carbon atom directly bonded to the OH
group of
the alcohol becomes oxidized (increases its bonds to oxygen and
decreases
its bonds to hydrogen) and the chromium (VI) atom of the Jones
reagent
is reduced to Cr3+.
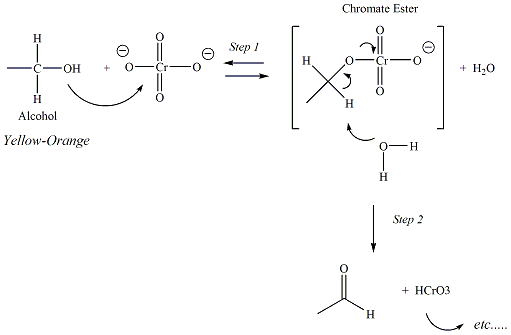
Chromium trioxide is an orange-colored or
red-orange reagent
which upon reduction
(reaction with primary or secondary alcohols) turns green or
blue-green. Over the course of the organic
oxidation, the Cr is reduced
to Cr (III). The first two steps of
the
reaction mechanism help to explain why tertiary alcohols do not
undergo
oxidation with the Jones reagent. In
step 2, water reacts with a proton of the chromate ester that is
bonded
to the
carbon atom of the former alcohol functional group.
Tertiary alcohols do not have a hydrogen atom
bonded to this carbon, therefore the reaction could not proceed
beyond
this
point