The separatory funnel is used
to perform simple extractions. The separatory funnel has three
distinct parts: 1) the stopper (at the top), 2) the vessel
itself, and 3) the stopcock (at the bottom). When the separatory
funnel is in use, it is generally positioned through a ring (of
appropriate size) mounted to a sturdy ring stand (Figure 1 )
|
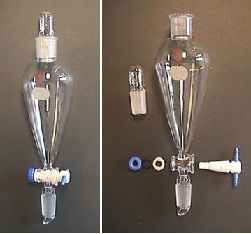
Figure 1
|
|
The separatory funnel is used to perform simple extractions.
The separatory funnel has three distinct parts: 1) the
stopper (at the top), 2) the vessel itself, and 3) the
stopcock (at the bottom) (Figure 1) When the separatory
funnel is in use, it is generally positioned through a ring
(of appropriate size) mounted to a sturdy ring stand. The
solvents and
solutions used in the extraction are added to the separatory
funnel through the ground-glass joint at the top of the
funnel. Solvents and solutions should be added to the
funnel using a glass funnel to avoid contaminating the
joint. (Contaminants will prevent a tight seal from
forming). The stopcock at the bottom of the funnel
must be in the closed position (perpendicular to the stem or
"drain"). When two immisible solvents (solvents
insoluble in each other) are placed in the separatory
funnel, two distinct layers should be visible.
(Figure 2). |
|

Figure 2
|
|
|
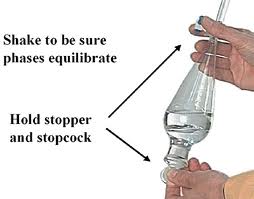
Figure 3
|
|
The
solvents/solutions are typically an organic (lipohilic,
non-polar) phase and an aqueous (hydrophilic, polar)
phase. These phases are not misible. Shaking the
separatory funnel allows the two solvents to mix and
provides the opportunity for components dissolved in one of
the solvents to be transferred to the other solvent.
Proper technique for shaking the separatory funnel is
illustrated in Figure 2. The stopper should be placed
in the top of the funnel before removing it from the ring
and the stopcock should be in the closed position. The
stopper must be held firmly in place by holding it with the
index finger or palm of the hand. The stem (out spout)
must be directed into the back of the hood and the funnel
should be positioned with the stem pointed upward at an
angle of ~ 45degrees. Mixing occurs by gently shaking
the funnel for ~10-30 seconds, after which the stopcock
should be opened, with the funnel tilted upward, to
vent any gases generated by the shaking process. After
venting the gases, the stopcock should be closed and the
funnel returned to the ring.
The two layers are separated after shaking by draining the
lower layer from the bottom of the separatory funnel.
The stopper must be removed from the funnel prior to
draining. The bottom layer is drained into a clean,
labeled beaker or flask by opening the stopcock and closing
it quickly when the interface region reaches the bottom of
the funnel. A second beaker or flask is used to
collect the remaining layer in the funnel.
|
|

Figure 4
|
|
The mixture in the funnel will be cloudy when the two immiscible
solvents mix during shaking. The two distinct solvent layers
will separate out after the funnel is allowed to sit undisturbed
in the ring for a few moments. Sometimes emulsions form and
the two layers do not separate cleanly. emulsions are
mixtures of two immiscible solvents where one solvent becomes
encapsulated ("trapped") by the other solvent. Techniques
have been developed to help break emulsions that form during the
extraction process and are summarized Table 1. Be aware that
breaking an emulsion is often tricky and requires a bit of
finesse. one or possibly all of the techniques listed in
Table 1 may be necessary to break the emulsion.
| Addition of small quantities (10-100mg) of salt (NaCl or
equivalent) to the funnel, followed by gently shaking or
swirling. Adding too much salt to the
separatory funnel may clog the stopcock, so add sparingly. |
| Addition of one or the other of solvents used in the
extraction. |
| Addition of ethanol which has solubility in both aqueous
and organic solvents. |
| Addition of small quantities of dilute aqueous acid or
base (1-2ml). Note that some components may react
with acid or base so use this method as a last resort if
the components are acid or base sensitive. |
Table 1: Techniques for breaking emulsions
In addition to emulsions, separation of the two solvents is often
complicated by a "third layer" or the interface between
the two solvents. A large interface is likely just a small
emulsion and can be handled using the techniques listed in table
1. Usually the best technique to use for an intereface
problem is to add on or the other of the two solvents used in the
extraction. The interface contains both solvents and likely
all the components of the mixture. If the interface is
collected with a specific layer, that layer will be contaminated
with all components of the mixture. To ensure a
completely clean separation of the two layers, it is sometimes
nessary to collect the interface in a third beaker.



