Organic Chemistry Laboratory II
Comparison of Substitution and Elimination Reactions
Experiment Description and Background
Description
In this two-week experiment, students will work in pairs to react an alkyl bromide (substrate) with and alkoxide (nucleophile or base) to determine the ratio of substitution products (ether) relative to elimination products (alkene(s)). Elimination and substitution reactions (E1,E2, SN1, SN2) occur through unique mechanisms that are dependent on the reaction conditions, the substitution of the reacting carbon of the substrate (i.e., the carbon atom bonded to the leaving group or Br in this case) and the structural features of the nucleophile or base (alkoxide in this case) used in the reaction. Substitution reactions often compete with elimination reactions under certain reaction conditions and with certain substrates. In this experiment, the effects of structural features of the alkyl bromide and the alkoxide on the outcome of the reaction will be evaluated.
Each bench in the lab will be assigned specific reactions (see table below), the product(s) will be analyzed by chemical tests and gas chromatographic-masss spectrometric (GC-MS) analysis. Students will conduct the chemical tests on their reaction products and known compounds. The GC-MS data will be provided. The effect of alkyl bromide substitution and structure of the alkoxide will be determined by compiling results from all of the groups.
In this two-week experiment, students will work in pairs to react an alkyl bromide (substrate) with and alkoxide (nucleophile or base) to determine the ratio of substitution products (ether) relative to elimination products (alkene(s)). Elimination and substitution reactions (E1,E2, SN1, SN2) occur through unique mechanisms that are dependent on the reaction conditions, the substitution of the reacting carbon of the substrate (i.e., the carbon atom bonded to the leaving group or Br in this case) and the structural features of the nucleophile or base (alkoxide in this case) used in the reaction. Substitution reactions often compete with elimination reactions under certain reaction conditions and with certain substrates. In this experiment, the effects of structural features of the alkyl bromide and the alkoxide on the outcome of the reaction will be evaluated.
Each bench in the lab will be assigned specific reactions (see table below), the product(s) will be analyzed by chemical tests and gas chromatographic-masss spectrometric (GC-MS) analysis. Students will conduct the chemical tests on their reaction products and known compounds. The GC-MS data will be provided. The effect of alkyl bromide substitution and structure of the alkoxide will be determined by compiling results from all of the groups.
NUCLEOPHILIC SUBSTITUTIONS
There are two distinct mechanisms through which nucleophilic substitution reactions occur, referred to as SN1 and SN2. Alkyl halides react in nucleophilic substitutions where the sp3 carbon bonded to the halogen serves as the electrophile (E+), and the halogen serves as the leaving group (LG). Other types of functional groups (tosylates and alcohols) may also undergo nucleophilic substitution reactions through a slightly modified mechanism.
SN1 Substitutions
General Mechanism
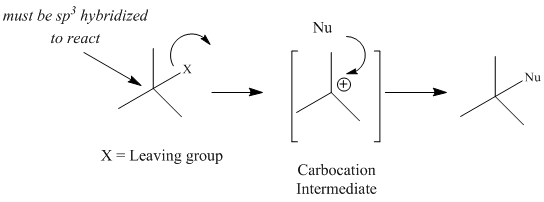
SN1 nucleophilic substitutions typically occur with secondary (2°) and tertiary (3°) alkyl halides, alcohols or tosylates, or allylic halides, alcohols or tosylsates with primary (1°), 2° or 3° degrees of alkyl substitution. For alkyl halides, the leaving group (LG) is the halide ion, for alcohols the LG is water or another activated oxoium ion, and for tosylates the LG is the tosyl ion. The reaction is a multistep reaction that involves the formation of a carbocation intermediate. The carbocation is generated when the leaving group bonded to the sp3 carbon breaks. Formation of the carbocation intermediate is the rate-determining step of the reaction. For alkyl halides, the reaction is a two-step reaction.
Factors Affecting Reaction Rates The name "SN1"
is intended to describe the reaction type and the kinetics
associate with the reaction. The "S" refers to
substitution, the "N" refers to nucleophilic, and the "1"
indicates that the reaction is first-order. First order
reactions are those whose reaction rates (i.e. how fast the
reaction occurs) are dependent on the concentration of only
one reaction species. For SN1 reactions,
the rate of the reaction is only dependent on the
concentration of reaction species involved in the
rate-determining step of the reaction, in this case, the
concentration of the alkyl halide. The rate at which
an SN1 reaction occurs will be determined by the
stability of the transition state (TS) leading to the
carbocation generated from the alkyl or allylic
halide. Highly substituted alkyl halides react faster
than less substituted alky halides because their
corresponding transition states (TS), leading to the
carbocations are more stable. (Rate: 3 ° > 2 ° >
1°). The more stable the carbocations, the lower the
transition state leading to formation of the carbocation
(Hammond Postulate) and the faster the overall reaction.
SN2 Substitutions
General Mechanism
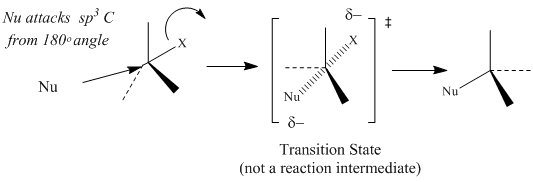
SN2 nucleophilic substitutions typically occur with 1° and 2° alkyl halides, activated alcohols or tosylates, or allylic halides, activated alcohols or tosylsates with 1° or 2° degrees of alkyl substitution. For alkyl halides, the leaving group is the halide ion, for alcohols the leaving group is an activated oxoium ion, and for tosylates the leaving group is the tosyl ion. The reaction is a single-step reaction that does not involve the formation of a carbocation intermediate. The one-step reaction involves the formation of a transition state whose structure is described by simultaneous bond-breaking of sp3 carbon-halogen bond and bond formation between the nucleophile and the sp3 carbon. The single step of the reaction is the rate-determining step.
There are two distinct mechanisms through which nucleophilic substitution reactions occur, referred to as SN1 and SN2. Alkyl halides react in nucleophilic substitutions where the sp3 carbon bonded to the halogen serves as the electrophile (E+), and the halogen serves as the leaving group (LG). Other types of functional groups (tosylates and alcohols) may also undergo nucleophilic substitution reactions through a slightly modified mechanism.
SN1 Substitutions
General Mechanism
SN1 nucleophilic substitutions typically occur with secondary (2°) and tertiary (3°) alkyl halides, alcohols or tosylates, or allylic halides, alcohols or tosylsates with primary (1°), 2° or 3° degrees of alkyl substitution. For alkyl halides, the leaving group (LG) is the halide ion, for alcohols the LG is water or another activated oxoium ion, and for tosylates the LG is the tosyl ion. The reaction is a multistep reaction that involves the formation of a carbocation intermediate. The carbocation is generated when the leaving group bonded to the sp3 carbon breaks. Formation of the carbocation intermediate is the rate-determining step of the reaction. For alkyl halides, the reaction is a two-step reaction.
|
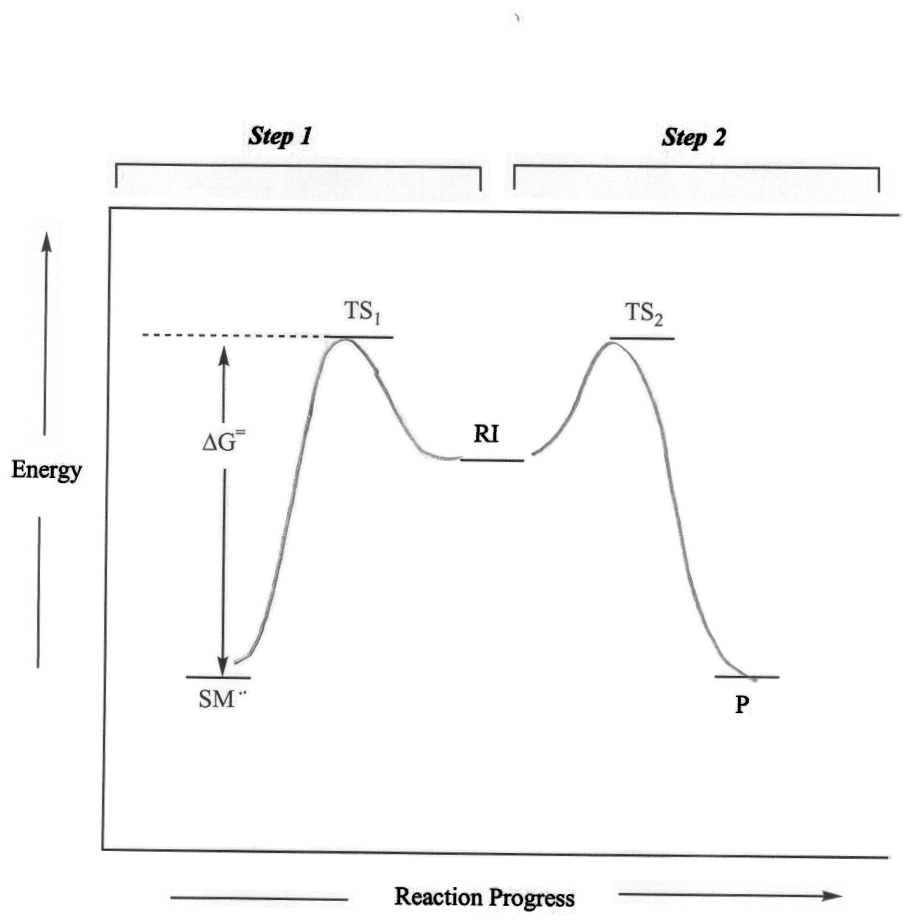 |
Figure 1: General SN1 Mechanism and Reaction Energy Diagram
SN2 Substitutions
General Mechanism
SN2 nucleophilic substitutions typically occur with 1° and 2° alkyl halides, activated alcohols or tosylates, or allylic halides, activated alcohols or tosylsates with 1° or 2° degrees of alkyl substitution. For alkyl halides, the leaving group is the halide ion, for alcohols the leaving group is an activated oxoium ion, and for tosylates the leaving group is the tosyl ion. The reaction is a single-step reaction that does not involve the formation of a carbocation intermediate. The one-step reaction involves the formation of a transition state whose structure is described by simultaneous bond-breaking of sp3 carbon-halogen bond and bond formation between the nucleophile and the sp3 carbon. The single step of the reaction is the rate-determining step.
 |
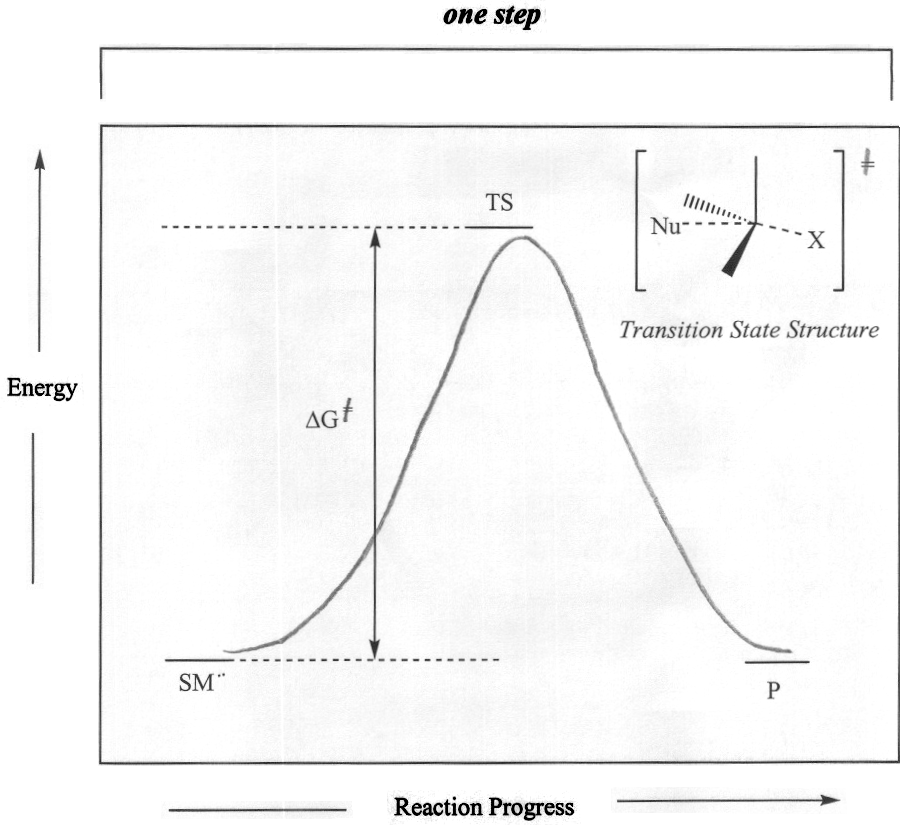 |
Figure 2: General SN2 Mechanism and Reaction Energy Diagram
Factors Affecting Reaction Rates
The name "SN2" is intended to describe the reaction type and the kinetics associate with the reaction. The "S" refers to substitution, the "N" refers to nucleophilic, and the "2" indicates that the reaction is second-order. Second order reactions are those whose reaction rates (i.e. how fast the reaction occurs) are dependent on the concentration of two reaction species. For SN2 reactions, the rate of the reaction is dependent on the concentration of the reaction species involved in the rate-determining step, in this case, the concentration of both the alkyl halide and the nucleophile. Increasing the concentration of either the alkyl halide or the nucleophile in the reaction will increase the overall rate of the reaction. The rate at which an SN2 reaction occurs will be determined by the stability of transition state. Highly substituted alkyl halides tend to produce relatively unstable transition states (high energy) due to extreme steric crowding around the reacting carbon. (Rate: 1 ° > 2 ° > 3°). The more stable the transition state leading, the lower the activation energy of the rate-determining step and the faster the overall reaction.
ELIMINATIONS
There are also two distinct mechanisms for elimination reactions, E1 and E2. Alkyl halides react in elimination reactions where the electrons associated with the bond between the sp3 carbon bonded to the halogen and a hydrogen bonded to an adjacent sp3 carbon are used to form a new pi bond and H-X. Other types of functional groups (tosylates and alcohols) may also undergo nucleophilic substitution reactions through slightly modified mechanisms. Elimination reactions often complete with nucleophilic substitution reactions, depending on the substrate and reaction conditions involved.
E1 Eliminations
E1 eliminations always involve a carbocation intermediate.
Analysis and Characterization of Reaction Products
Analysis of reaction products typically includes a combination of chromatographic and spectroscopic techniques. Thin layer chromatography and gas chromatography, coupled with proton NMR and mass spectral data are used for this purpose in this experiment. Chemical tests can also be used to distinguish between alkyl bromide starting materials, ether and alkene products.
Gas Chromatography
Gas chromotography is an analytical method used to separate organic compounds based primarily on differences in boiling points. The method is effective for organic compounds with molecular weights less than 500g/mol. Since gas chromatography typically requires heating the to temperature in excess of 100°C, this method is not suitable for analysis of organic compounds that decompose at high temperatures. Gas chromatography is run using an instrument called a gas chromatograph. Like other forms of chromatography, gas chromatography has a stationary phase and a mobile phase to accomplish separation of compounds. The stationary phase is a column, either a packed column or a capillary column, that is positioned inside an oven. The mobile phase in gas chromatography is also referred to as the "carrier gas", is an inert gas, typically helium or nitrogen that moves the compounds to be analyzed through the column. Other parts of the gas chromatograph are the injection port and the detector. The injection port is positioned at the opening of the column. The compounds to be analyzed (analytes) are introduced onto the column through the injection port. The analytes are moved through the column by the carrier gas (mobile phase) at different rates depending on their boiling points and polarity. As the compounds of the mixture exit the end of the column, they are delivered to the detector which is positioned at the end of the column. The detector then sends information to a recorder (computer) to generate the chromatogram. The output of the gas chromatograph is referred to as a chromatogram. The chromatogram is a plot of the retention time (rt) along the x-axis and the amplitude along the y-axis. Detected compounds appear on the plot as peaks. Each peak corresponds to each component of the analyzed mixture, and peaks are cleanly separated or resolved. The area under each peak can be correlated to the concentration of the component in the sample mixture.
Mass Spectrometry (click for more information)
Mass spectrometry is an analytical technique used for determining the molecular weight of organic compounds. Mass spectrometry also detects the presence of significant isotopes of atoms in molecules, most notably, bromine and chlorine. The reactants and products in this experiment have unique molecular weights and are readily distinguished by mass spectrometry. Furthermore, the alkyl bromide starting materials contain a bromine atom which has a characteristic distribution of the 79Br (51%) and 81Br (49%) isotopes in the mass spectrum. Proton NMR spectroscopy provides information about the carbon skeleton of organic compounds. The primary and secondary alkyl bromides contain characteristic protons associated with the carbon bonded to the bromine atom. The alkene and ether products also contain characteristic protons that can be distinguished by proton NMR spectroscopy.
Chemical Tests
Chemical tests are reactions used to identify or verify the presence of a specific functional group in an organic compound. Chemical tests for a specific functional group are run along with a known compound that is used as a positive or negative control. For alkyl halides, multiple chemical tests are used to distinguish between primary, secondary and tertiary carbons. For chemical tests to be useful, the unknown (and known compounds) must be soluble in the chemical test reagent(s), otherwise false negative results for that functional group may occur. Some classification tests will not work as predicted and vary from unknown to unknown. Tips and warnings for interpreting the results of each classification test are provided with the procedures. Four chemical tests will be used in this experiment.
The name "SN2" is intended to describe the reaction type and the kinetics associate with the reaction. The "S" refers to substitution, the "N" refers to nucleophilic, and the "2" indicates that the reaction is second-order. Second order reactions are those whose reaction rates (i.e. how fast the reaction occurs) are dependent on the concentration of two reaction species. For SN2 reactions, the rate of the reaction is dependent on the concentration of the reaction species involved in the rate-determining step, in this case, the concentration of both the alkyl halide and the nucleophile. Increasing the concentration of either the alkyl halide or the nucleophile in the reaction will increase the overall rate of the reaction. The rate at which an SN2 reaction occurs will be determined by the stability of transition state. Highly substituted alkyl halides tend to produce relatively unstable transition states (high energy) due to extreme steric crowding around the reacting carbon. (Rate: 1 ° > 2 ° > 3°). The more stable the transition state leading, the lower the activation energy of the rate-determining step and the faster the overall reaction.
ELIMINATIONS
There are also two distinct mechanisms for elimination reactions, E1 and E2. Alkyl halides react in elimination reactions where the electrons associated with the bond between the sp3 carbon bonded to the halogen and a hydrogen bonded to an adjacent sp3 carbon are used to form a new pi bond and H-X. Other types of functional groups (tosylates and alcohols) may also undergo nucleophilic substitution reactions through slightly modified mechanisms. Elimination reactions often complete with nucleophilic substitution reactions, depending on the substrate and reaction conditions involved.
E1 Eliminations
E1 eliminations always involve a carbocation intermediate.
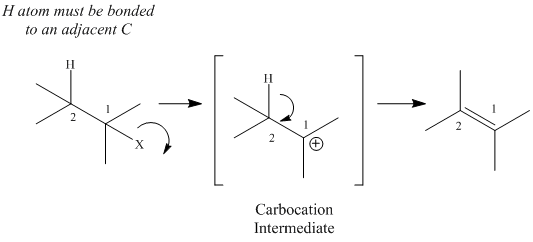 Mechanism of E1 Elimination |
|
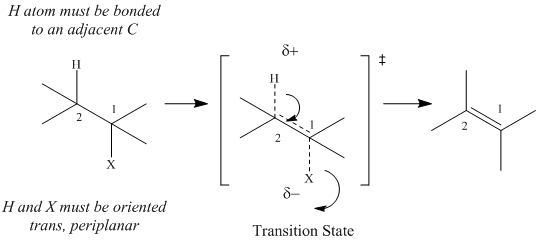 Mechanism of E2
Elimination
|
E2 Eliminations
Analysis and Characterization of Reaction Products
Analysis of reaction products typically includes a combination of chromatographic and spectroscopic techniques. Thin layer chromatography and gas chromatography, coupled with proton NMR and mass spectral data are used for this purpose in this experiment. Chemical tests can also be used to distinguish between alkyl bromide starting materials, ether and alkene products.
Gas Chromatography
Gas chromotography is an analytical method used to separate organic compounds based primarily on differences in boiling points. The method is effective for organic compounds with molecular weights less than 500g/mol. Since gas chromatography typically requires heating the to temperature in excess of 100°C, this method is not suitable for analysis of organic compounds that decompose at high temperatures. Gas chromatography is run using an instrument called a gas chromatograph. Like other forms of chromatography, gas chromatography has a stationary phase and a mobile phase to accomplish separation of compounds. The stationary phase is a column, either a packed column or a capillary column, that is positioned inside an oven. The mobile phase in gas chromatography is also referred to as the "carrier gas", is an inert gas, typically helium or nitrogen that moves the compounds to be analyzed through the column. Other parts of the gas chromatograph are the injection port and the detector. The injection port is positioned at the opening of the column. The compounds to be analyzed (analytes) are introduced onto the column through the injection port. The analytes are moved through the column by the carrier gas (mobile phase) at different rates depending on their boiling points and polarity. As the compounds of the mixture exit the end of the column, they are delivered to the detector which is positioned at the end of the column. The detector then sends information to a recorder (computer) to generate the chromatogram. The output of the gas chromatograph is referred to as a chromatogram. The chromatogram is a plot of the retention time (rt) along the x-axis and the amplitude along the y-axis. Detected compounds appear on the plot as peaks. Each peak corresponds to each component of the analyzed mixture, and peaks are cleanly separated or resolved. The area under each peak can be correlated to the concentration of the component in the sample mixture.
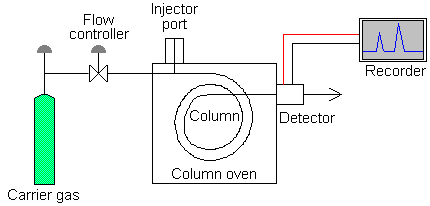 Schematic Diagram of a Gas
Chromatograph
|
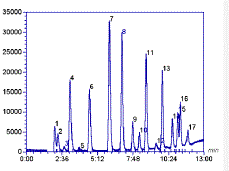 An Example Gas Chromatogram |
Mass Spectrometry (click for more information)
Mass spectrometry is an analytical technique used for determining the molecular weight of organic compounds. Mass spectrometry also detects the presence of significant isotopes of atoms in molecules, most notably, bromine and chlorine. The reactants and products in this experiment have unique molecular weights and are readily distinguished by mass spectrometry. Furthermore, the alkyl bromide starting materials contain a bromine atom which has a characteristic distribution of the 79Br (51%) and 81Br (49%) isotopes in the mass spectrum. Proton NMR spectroscopy provides information about the carbon skeleton of organic compounds. The primary and secondary alkyl bromides contain characteristic protons associated with the carbon bonded to the bromine atom. The alkene and ether products also contain characteristic protons that can be distinguished by proton NMR spectroscopy.
Chemical Tests
Chemical tests are reactions used to identify or verify the presence of a specific functional group in an organic compound. Chemical tests for a specific functional group are run along with a known compound that is used as a positive or negative control. For alkyl halides, multiple chemical tests are used to distinguish between primary, secondary and tertiary carbons. For chemical tests to be useful, the unknown (and known compounds) must be soluble in the chemical test reagent(s), otherwise false negative results for that functional group may occur. Some classification tests will not work as predicted and vary from unknown to unknown. Tips and warnings for interpreting the results of each classification test are provided with the procedures. Four chemical tests will be used in this experiment.
| Chemical
Test |
Description |
| Sodium Iodide in
Acetone |
This is a chemical test for primary
and secondary alkyl bromides. Tertiary alkyl
bromides and sometimes secondary alkyl bromide,
react slowly or not at all. Ethers and alkenes do
not react. |
| Silver Nitrate
in Ethanol |
This is a chemical test, primarily
for tertiary alkyl bromides. Tertiary alkyl
bromides react relatively quickly compared to
secondary and primary alkyl bromides. Ethers
and alkenes do not react. Occasionally,
secondary and primary alkyl bromides give false
positive results |
| KMnO4
Test |
This is a chemical test for
alkenes. Ethers and alkyl bromides do not
react |
| Bromination Test |
This is a test for alkenes.
Ethers and alkyl bromides do not react. |