Organic Chemistry II | Lecture | Laboratory
Organic Chemistry Laboratory II
 |
|
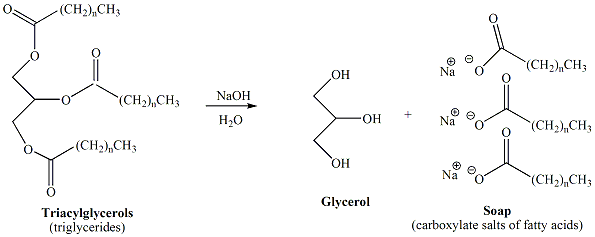
Each student will prepare approximately 5g of
soap.
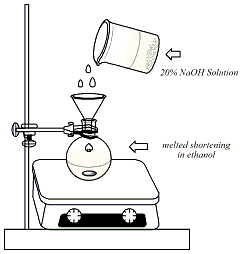
Weigh out 5g of
vegetable shortening into a 50-ml round-bottom
flask. Add 5 ml of ethanol and 5 ml of 20% (5M)
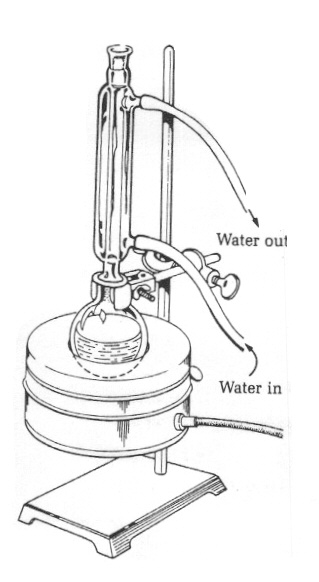
NaOH. Clamp the round bottom flask to a vertical
bar. Fit the flask with a heating mantle above a
magnetic stirplate. Grease the joint with a
minimal amount of shortening. Insert a magnetic
stir bar into the round-bottomed flask. |
|
 |
 |
|
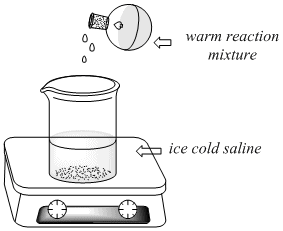
While the shortening/NaOH mixture is refluxing,
measure out 50 ml of 28% NaCl (saline) into a 150ml
beaker in a plastic ice bath. After the 15
minutes, disassemble the reflux apparatus
and, using a Hot Hands to hold the warm flask,
carefully pour the warm reaction mixture (including the
stir bar) into the beaker containing the ice cold
saline. Immediately place the beaker and ice bath
on the stir plate.
Stir vigorously for 5 minutes. A white
precipitate (soap) should form. Allow the mixture to
stir vigorously while setting up a vacuum filtration
apparatus.
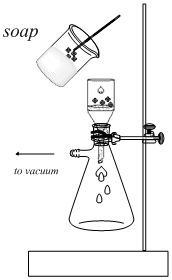
Clamp the flask to a vertical bar and insert a Buchner funnel fitted with a piece of filter paper into the flask. Place 30ml of 28% NaCl (saline) in a 150 ml flask in the ice bath. Use ~10ml of this cold 28% NaCl (saline) to wet the filter paper. Turn on the vacuum and pour the soap mixture onto the Buchner funnel. Rinse the soap with the remaining 20ml of ice cold 28% NaCl (saline). Then wash the soap once with 20ml of cold ethanol. Watch the volume of liquid in the filtration flask and empty it periodically if it exceeds half the volume of the flask. |
|
 |
Transfer the washed soap to weigh boat labeled with
your name and date. Place
the granular soap in the container at the instructor’s bench
for week 2.
| Tube |
Mixture |
Observation |
Observations after addition
of sodium phosphate |
| 1 |
Soap + CaCl2 |
||
| 2 |
Soap + MgCl2 |
||
| 3 |
Soap + FeCl3 |
||
| 4 |
Detergent + CaCl2 |
||
| 5 |
Detergent + MgCl2 |
||
| 6 |
Detergent + FeCl3 |
||
| 7 |
DI Water + CaCl2 | ||
| 8 |
DI Water + MgCl2 | ||
| 9 |
DI Water + FeCl3 |