Organic Chemistry Laboratory II
Kinetics of the SN1
Reaction: Hydrolysis of Alkyl Chlorides to Alcohols
Experiment Background
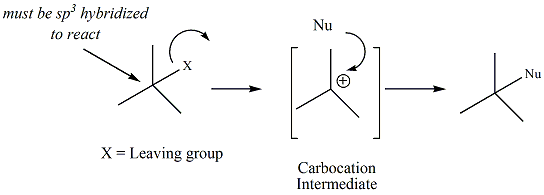
Nucleophilic Substitutions
There are two distinct mechanisms through
which nucleophilic substitution reactions occur, referred to as SN1
and SN2.
Alkyl
halides react in nucleophilic substitutions where the sp3 carbon
bonded
to the halogen serves as the electrophile (E+), and the halogen
serves as the leaving group (LG). Other types of functional
groups (tosylates and alcohols) may also undergo nucleophilic
substitution reactions througfh a slightly modified mechanism.
SN1 Nucleophilic
Substitution
 |
 |
The rate-determining step (rds) of the reaction is the
step that involves formation of the carbocation.
When alkyl halides are the starting materials, this step
is the first step of the reaction. The overall rate of
the reaction (i.e., how much time is required to
complete all steps in the entire
reaction) is dependent on how long it takes for the rds
to occur. The duration of the rds is associated
with the activation energy (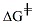 )of this
step, which in turn is dependent on the stability
(energy) of the carbocation intermediate. A less
stable or higher energy carbocation, has a higher
transition state (TS) energy and a correspondingly
larger activation energy ( )of this
step, which in turn is dependent on the stability
(energy) of the carbocation intermediate. A less
stable or higher energy carbocation, has a higher
transition state (TS) energy and a correspondingly
larger activation energy (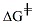 ).
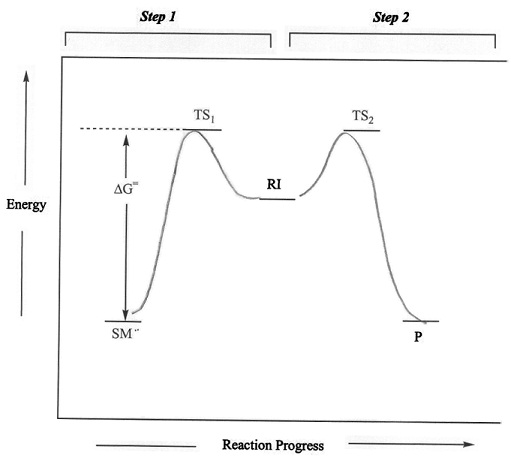
We know from previous studies that the stability of
carbocations follow the trends shown in Figure 2. Thus,
reactions that generate more stable carbocations,
generally occur faster than those involving less stable
carbocations. A reaction energy diagram
illustrating the relative activation energies associated
with carbocations of different stabilities. ).
We know from previous studies that the stability of
carbocations follow the trends shown in Figure 2. Thus,
reactions that generate more stable carbocations,
generally occur faster than those involving less stable
carbocations. A reaction energy diagram
illustrating the relative activation energies associated
with carbocations of different stabilities.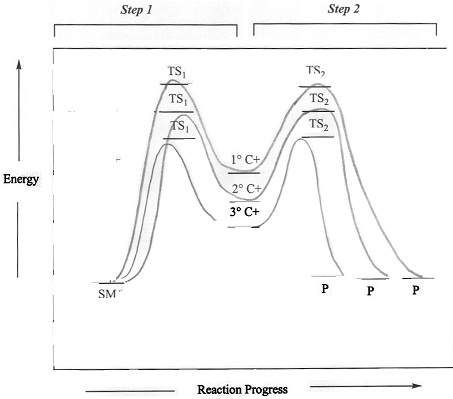 Figure 3: Reaction energy diagram of an SN1 reaction involving primary, secondary and teriary carbocations. |
|
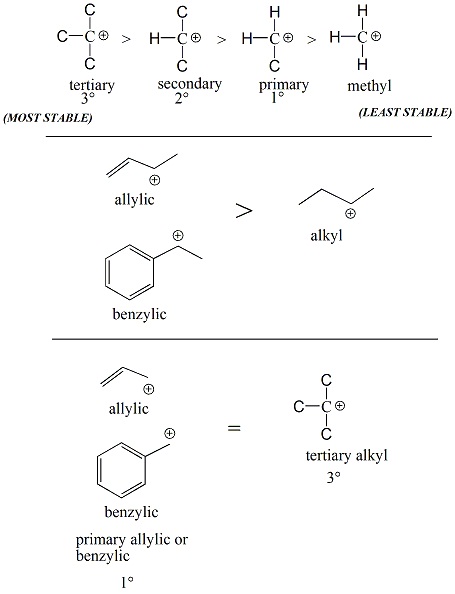 Figure 2: Relative Stability of Alkyl, Allylic and Benzylic Carbocations |
|
The two alkyl halides used in this experiment; 1-chloroethylbenzene and 2-phenyl-2-propylchloride. The 1-chlorobenzene is a secondary benzylic halide and 2-phenyl-2-propylchloride chloride is a tertiary benzylic halide. Each of these alkyl halides are treated with water to generate an alcohol product. The reaction is referred to as a "hydrolysis" since water ("hydro") is used to break the bond between the carbon and clorine atom ("lysis") in the starting material. The reactions occur through an SN1 mechanism and the rates of each reaction are evaluated in three different solvents with varying polarity and protic character. Based on the information above, 1-chlorobenzene would be predicted to react faster than tert-butyl chloride. The effect of the various solvents on the rates of these reactions are are harder to predict, but will be determined experimentally. The reaction schemes for the hydrolysis of tert-butyl chloride and 1-chloroethylbenzene are shown in Figure 4 on the right. An alcohol product is generated in each of these reactions along with hydrocholic acid (HCl). For every molecule of alcohol that is generated in the reaction, an equivalent number of molecules of HCl are generated. Thus, the conversion of the alkyl/benzylic halides to their corresponding alcohols can be monitored measuring the generation of HCl in the reaction. This can be done by titration with sodium hydroxide (NaOH). |
|
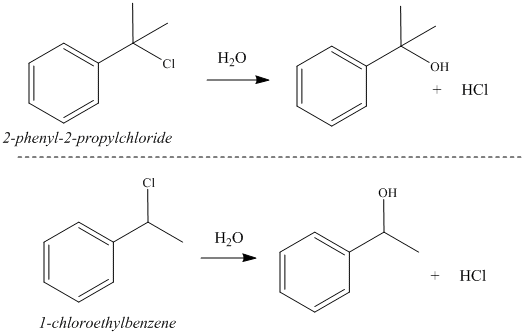 Figure 4:
Hydrolysis reactions of tert-butyl chloride and
1-chloroethylbenzene
|
|
|
Measuring the Rate of the Hydrolysis Reaction and Determining the Rate Constant The rate at which the alkyl or benzylic halide is converted to the alcohol is measured by titrating the HCl that is generated in the reaction with a 0.1M solution of NaOH. Bromothymol blue (BRB) is used as a pH indicator in this reaction. BRB is blue under basic conditions (pH 7.6 and higher) and yellow under neutral or acidic conditions (pH 6.0 or lower). At the start of the reaction (t =0), 1.5mL of NaOH (0.1M) is added to the flask that contains the BRB indicator and the aqueous reaction solvent. The nucleophile (H2O) is derived from the solvent. Under basic conditions (pH 7.6 or higher), the BRB maintains a blue color. When the alkyl or benzylic halide is added, it reacts with the water in the solvent and releases HCl. The released, acidic HCl reacts with any NaOH in the solution. As the reaction continues, an excess of HCl is generated and the pH of the solution is lowered and the color of the solution shifts from blue to green and then yellow. More NaOH is added to continuously react with HCl as it is generated in the hydrolysis reaction. The time it takes for the color to change after addition of NaOH represents the time it takes for HCl to be generated which in turn represents the time it takes for the alkyl/benzylic halide to be converted to the alcohol. Watch a video here to observe a similar titration using BRB as indicator. The rate of the reaction is dependent on the concetration of the alkyl/benzylic halide and the rate constant (k) for a specific set of conditions (i.e., solvent, temperatuure) (Figure 5). The rate constant is determined in this experiment for three different reaction conditions (i.e., three different solvents) using the same concentration of alkyl or benzylic halide. A rate equation can be set up using the data generated in the titration of the reaction. |
|
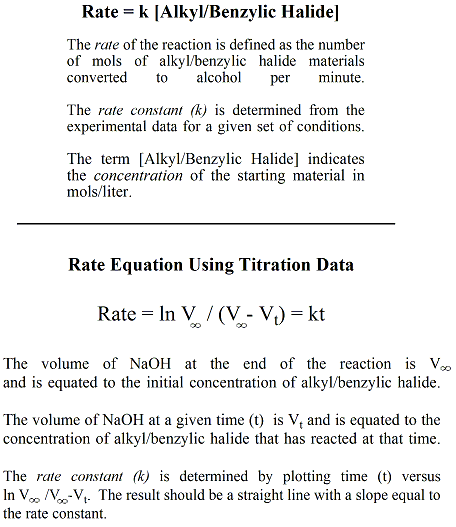 Figure 5: Rate Equations |
|