Organic Chemistry Laboratory II
Identification of Alcohols by Chemical
Methods
Experiment Description and Background
Experiment Summary
Alcohols: Structure and Physical Properties
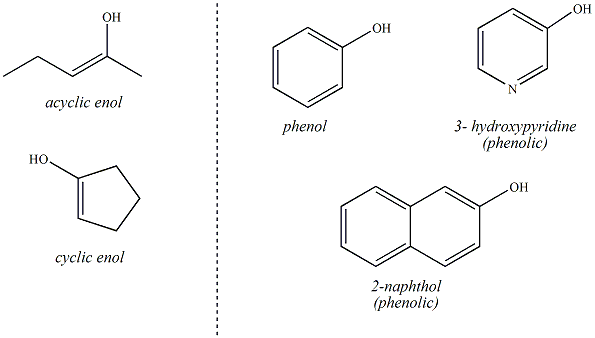
Alcohols are organic molecules that contain an sp3 hybridized carbon atom bonded to the oxygen atom of a hydroxyl group (OH). The alcohol may be designated as a primary secondary or tertiary alcohol depending on the alkyl substitution of the sp3 carbon bonded to the hydroxyl. If the sp3 carbon bonded to the hydroxyl is primary (bonded to only one other carbon atom), the alcohol is designated as a primary alcohol. If the sp3 carbon is secondary (bonded to two other carbons), the alcohol is secondary and if the carbon is tertiary (bonded to three other carbons), the alcohol is designated as a tertiary alcohol. Alcohols may be acyclic (as shown below) or the hydroxyl group may be bonded to an sp3 carbon of a hydrocarbon ring (cyclic alcohols, eg. cyclohexanol)

Compounds that contain a hydroxyl group bonded to an sp2
carbon are not considered to be alcohols. These compounds
are enols
(if the sp2 carbon is part of an alkene functional
group) or phenols
(if the sp2 carbon is part of a benzene ring;
compounds are considered to be phenolic if the sp2 carbon is part
of an aromatic compound that is not specifically benzene , i.e,
naphthalene or pyridine). This is an important distinction
because enols and phenols (and phenolic compounds) do no react
in the same way as alcohols. Enols may be acyclic (no
ring) or they may be cyclic (part of a ring).
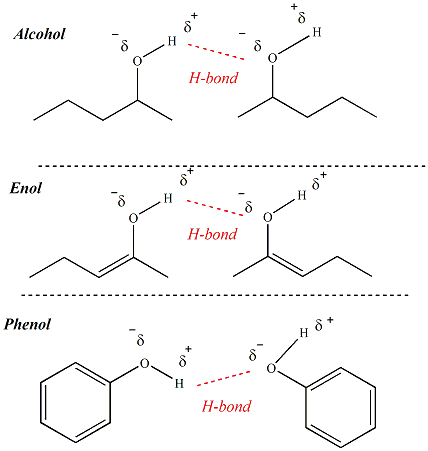
Primary, secondary and tertiary alcohols, as well as enols and phenols have similar physical properties, but different chemical properties. All alcohols, enols and phenols are capable of hydrogen bonding since all of these functional groups contain a hydrogen atom bonded to an electronegative oxygen atom. The polarized O-H bond of alcohols, enols and phenols allows for hydrogen bonding networks that influence physical properties such as boiling point (more hydrogen bonding results in higher boiling points).
Reactivity of Alcohols, Enols and Phenols
For all reactions involving alcohol functional groups, the reactivity of the alcohol functional group is focused on the hydroxyl group (OH) and/or the sp3 carbon bonded to the hydroxyl group.
Acid/Base Reactions of Alcohols, Enols and Phenols
In general, alcohols are not considered to be acidic or basic (pka of OH ~16-19) in physiological environments (pH ~7.4), however in some reactions involving alcohols that are run at either very high pH (basic) environments or very low pH environments (acidic), alcohols may either accept a proton (i.e., behave as a base) or donate a proton (behave as an acid). This behavior is referred to as amphoteric, meaning that alcohols having the ability to behave as either an acid or base in pH environments where ionization of the alcohol can occur. When an alcohol behaves as a base and accepts a proton, the resulting conjugate acid is referred to as an oxonium ion. When an alcohol behaves as an acid and donates a proton, the resulting conjugate base is referred to as an alkoxide ion. Oxonium ions and alkoxides are important reaction species in a number of reactions involving alcohols. Primary, secondary and tertiary alcohols all may be amphoteric and all will form either oxonium ions or alkoxides ions in the right pH environment.
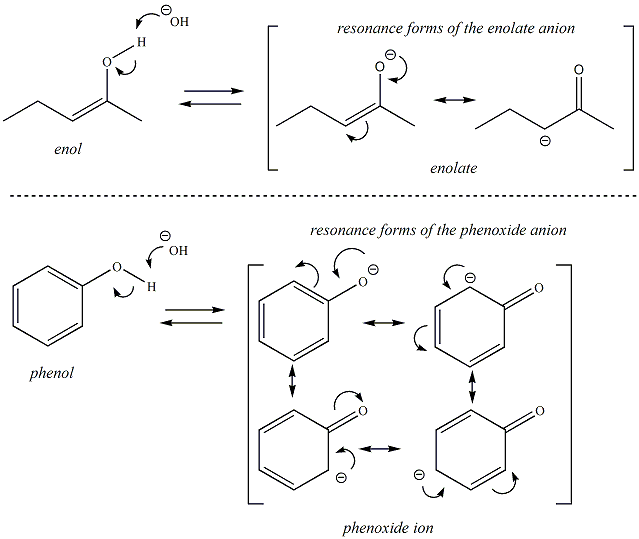
Enols and phenols are not amphoteric, they tend to be acidic (pKas ~ 8-10). When an enol behaves as an acid (high pH or basic environment) the resulting conjugate base is referred to as an enolate. An enolate has resonance forms where the negative charge may reside on either the oxygen (i.e., the original conjugate base) or the charge may be delocalized onto the carbon atom. The conjugate base of a phenol is referred to as a phenoxide ion. The phenoxide ion is also stabilized through resonance (the charge may delocalize through the aromatic ring).
Oxidations of Alcohols
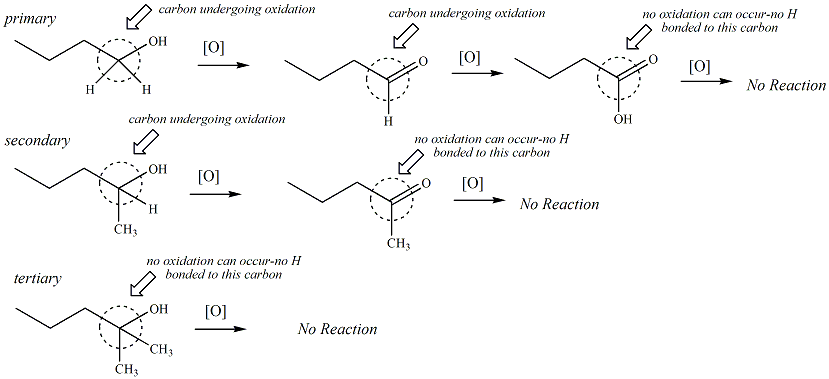
The carbon atom of primary and secondary alcohols are susceptible to oxidation, but tertiary alcohols are not. In order for oxidation to occur, the carbon atom of the alcohol must be bonded to at least one hydrogen. Oxidation refers to a chemical reaction that changes the oxidation state of an atom. For alcohols, oxidation refers to the oxidation state of the carbon atom bonded to the hydroxyl group. An oxidation is defined as a chemical reaction where the number of bonds between the carbon and hydrogen atoms decreases or the number of bonds between the carbon and oxygen atoms increases. The carbon atom of a primary alcohol (one C-O bond and two C-H bonds) may be oxidized to an aldehyde (two C-O bonds, one C-H bond) and then the resulting aldehyde may be oxidized again to a carboxylic acid (three C-O bonds, no C-H bonds). For secondary alcohols, the carbon atom may be oxidized to a ketone functional group, but does not get oxidized any further. The carbon of a primary alcohol is bonded to two hydrogens, the carbon atom of a secondary alcohol is bonded to one hydrogen and an aldehyde carbon (i.e., the carbonyl carbon) is bonded to one hydrogen. All of these functional groups can undergo oxidation. Tertiary alcohols, ketones and carboxylic acids cannot undergo oxidation because these functional groups do not have the required C-H bond.
Jones Test
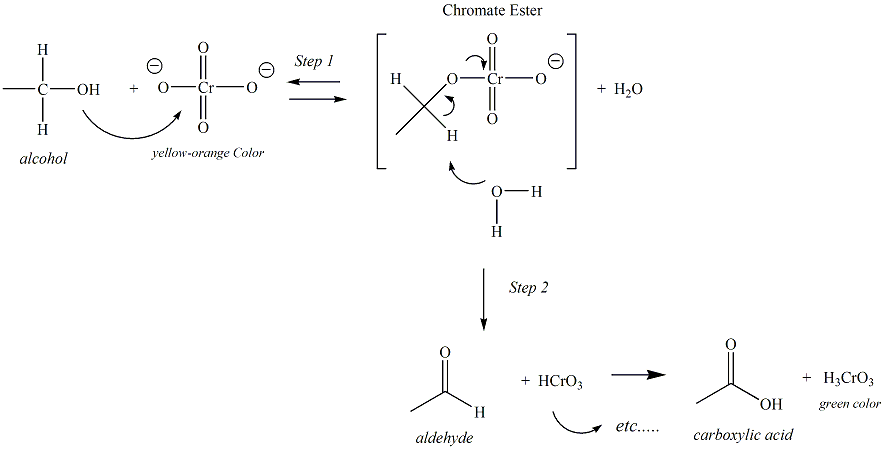
The Jones reagent is chromium trioxide (CrO3) in sulfuric acid (H2SO4). It is a potent oxidizing agent which rapidly oxidizes primary alcohols and aldehydes to carboxylic acids, and secondary alcohols to ketones. Alcohols react with the yellow-orange Jones reagent containing a Cr (VI) atom. Over the course of the organic oxidation, the Cr (VI) is reduced to Cr (III). The first two steps of the reaction mechanism help to explain why tertiary alcohols do not undergo oxidation with the Jones reagent. In step 2, water reacts with a proton of the chromate ester that is bonded to the carbon atom of the former alcohol functional group. Tertiary alcohols do not have a hydrogen atom bonded to this carbon, therefore the reaction could not proceed beyond this point. The oxidation mechanism of the Jones reagent with a primary alcohol is given below.
Iodoform Test
The iodoform reagent is a mixture of iodine (I2) and potassium iodide (KI). This test is generally used to test for methylketones, ketones where one of the two alkyl groups bonded to the carbonyl carbon is a methyl group. Reaction of a methylketone with strong base promotes the formation of an enolate which reacts with the electrophilic I2 to generate an iodomethylketone. Addition of two more equivalents of base and I2 lead to formation of the a-triiodoketone. Hydroxide ion then reacts with the carbonyl carbon of the ketone in a nucleophilic acyl substitution, liberating iodoform (CHI3) from the reaction mixture. Iodoform is not soluble in the reaction conditions and precipitates, indicating the reaction has occurred. Only methyl ketones (not other types of ketones) can undergo the iodoform reaction because only methyl ketones have three hydrogen atoms on the a carbon which are necessary to form the iodoform product.
Students will work
individually to characterize the reaction behavior of one
hydroxyl-containing compound (alcohol, phenol) using five
different chemical tests. Each bench in the lab will be
stocked with four compounds, one for each student working at
the bench. The compounds at the bench may be a primary
alcohol, a secondary alcohol, a tertiary alcohol, or a
phenol. There may be one or more of each type of
compound at each bench. Each student will be required to
share their results with all students at the bench, and all students are required to
report all the results from his or her bench.
Compounds will be labeled with the bench letter
and 1, 2, 3, and 4 corresponding to the alcohol. (eg.,
alcohols at bench A will be labeled as A-1, A-2, A-3 and
A-4. Please make sure
you return your alcohol to the proper bench!).
Each student at the bench will use one of the four alcohols
provided and test the alcohol using all five of the
following chemical tests:
- Jones Test
- Lucas Test
- Iodoform Test
- KMnO4 Test
- Br2/H2O Test
Alcohols: Structure and Physical Properties
Alcohols are organic molecules that contain an sp3 hybridized carbon atom bonded to the oxygen atom of a hydroxyl group (OH). The alcohol may be designated as a primary secondary or tertiary alcohol depending on the alkyl substitution of the sp3 carbon bonded to the hydroxyl. If the sp3 carbon bonded to the hydroxyl is primary (bonded to only one other carbon atom), the alcohol is designated as a primary alcohol. If the sp3 carbon is secondary (bonded to two other carbons), the alcohol is secondary and if the carbon is tertiary (bonded to three other carbons), the alcohol is designated as a tertiary alcohol. Alcohols may be acyclic (as shown below) or the hydroxyl group may be bonded to an sp3 carbon of a hydrocarbon ring (cyclic alcohols, eg. cyclohexanol)


Primary, secondary and tertiary alcohols, as well as enols and phenols have similar physical properties, but different chemical properties. All alcohols, enols and phenols are capable of hydrogen bonding since all of these functional groups contain a hydrogen atom bonded to an electronegative oxygen atom. The polarized O-H bond of alcohols, enols and phenols allows for hydrogen bonding networks that influence physical properties such as boiling point (more hydrogen bonding results in higher boiling points).

For all reactions involving alcohol functional groups, the reactivity of the alcohol functional group is focused on the hydroxyl group (OH) and/or the sp3 carbon bonded to the hydroxyl group.
Acid/Base Reactions of Alcohols, Enols and Phenols
In general, alcohols are not considered to be acidic or basic (pka of OH ~16-19) in physiological environments (pH ~7.4), however in some reactions involving alcohols that are run at either very high pH (basic) environments or very low pH environments (acidic), alcohols may either accept a proton (i.e., behave as a base) or donate a proton (behave as an acid). This behavior is referred to as amphoteric, meaning that alcohols having the ability to behave as either an acid or base in pH environments where ionization of the alcohol can occur. When an alcohol behaves as a base and accepts a proton, the resulting conjugate acid is referred to as an oxonium ion. When an alcohol behaves as an acid and donates a proton, the resulting conjugate base is referred to as an alkoxide ion. Oxonium ions and alkoxides are important reaction species in a number of reactions involving alcohols. Primary, secondary and tertiary alcohols all may be amphoteric and all will form either oxonium ions or alkoxides ions in the right pH environment.
|
When an alcohol
behaves as a base (in low pH or acidic
environments), the
atom of the hydroxyl group accepts a proton to generate an oxonium ion 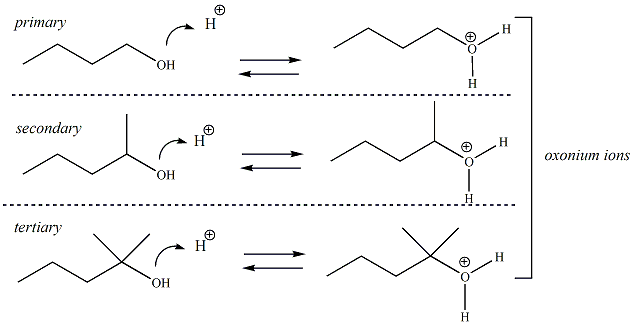 |
|
When an alcohol behaves
as an acid (in high pH or basic environments), the
hydrogen atom of the hydroxyl group donates a proton
to generate an alkoxide ion.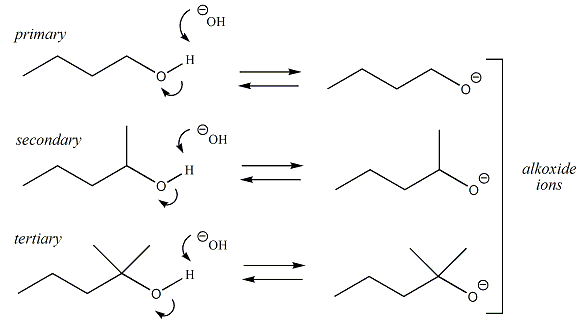 |
Enols and phenols are not amphoteric, they tend to be acidic (pKas ~ 8-10). When an enol behaves as an acid (high pH or basic environment) the resulting conjugate base is referred to as an enolate. An enolate has resonance forms where the negative charge may reside on either the oxygen (i.e., the original conjugate base) or the charge may be delocalized onto the carbon atom. The conjugate base of a phenol is referred to as a phenoxide ion. The phenoxide ion is also stabilized through resonance (the charge may delocalize through the aromatic ring).

Oxidations of Alcohols
The carbon atom of primary and secondary alcohols are susceptible to oxidation, but tertiary alcohols are not. In order for oxidation to occur, the carbon atom of the alcohol must be bonded to at least one hydrogen. Oxidation refers to a chemical reaction that changes the oxidation state of an atom. For alcohols, oxidation refers to the oxidation state of the carbon atom bonded to the hydroxyl group. An oxidation is defined as a chemical reaction where the number of bonds between the carbon and hydrogen atoms decreases or the number of bonds between the carbon and oxygen atoms increases. The carbon atom of a primary alcohol (one C-O bond and two C-H bonds) may be oxidized to an aldehyde (two C-O bonds, one C-H bond) and then the resulting aldehyde may be oxidized again to a carboxylic acid (three C-O bonds, no C-H bonds). For secondary alcohols, the carbon atom may be oxidized to a ketone functional group, but does not get oxidized any further. The carbon of a primary alcohol is bonded to two hydrogens, the carbon atom of a secondary alcohol is bonded to one hydrogen and an aldehyde carbon (i.e., the carbonyl carbon) is bonded to one hydrogen. All of these functional groups can undergo oxidation. Tertiary alcohols, ketones and carboxylic acids cannot undergo oxidation because these functional groups do not have the required C-H bond.

Jones Test
The Jones reagent is chromium trioxide (CrO3) in sulfuric acid (H2SO4). It is a potent oxidizing agent which rapidly oxidizes primary alcohols and aldehydes to carboxylic acids, and secondary alcohols to ketones. Alcohols react with the yellow-orange Jones reagent containing a Cr (VI) atom. Over the course of the organic oxidation, the Cr (VI) is reduced to Cr (III). The first two steps of the reaction mechanism help to explain why tertiary alcohols do not undergo oxidation with the Jones reagent. In step 2, water reacts with a proton of the chromate ester that is bonded to the carbon atom of the former alcohol functional group. Tertiary alcohols do not have a hydrogen atom bonded to this carbon, therefore the reaction could not proceed beyond this point. The oxidation mechanism of the Jones reagent with a primary alcohol is given below.

Iodoform Test
The iodoform reagent is a mixture of iodine (I2) and potassium iodide (KI). This test is generally used to test for methylketones, ketones where one of the two alkyl groups bonded to the carbonyl carbon is a methyl group. Reaction of a methylketone with strong base promotes the formation of an enolate which reacts with the electrophilic I2 to generate an iodomethylketone. Addition of two more equivalents of base and I2 lead to formation of the a-triiodoketone. Hydroxide ion then reacts with the carbonyl carbon of the ketone in a nucleophilic acyl substitution, liberating iodoform (CHI3) from the reaction mixture. Iodoform is not soluble in the reaction conditions and precipitates, indicating the reaction has occurred. Only methyl ketones (not other types of ketones) can undergo the iodoform reaction because only methyl ketones have three hydrogen atoms on the a carbon which are necessary to form the iodoform product.
Mechanism of the
iodoform reaction of a methyl ketone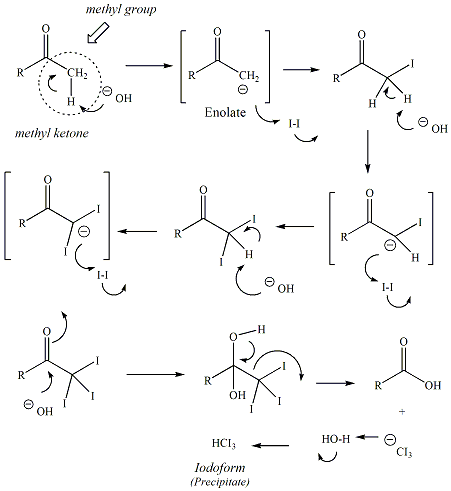 |
|
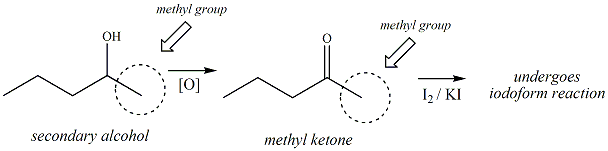
Some
secondary alcohols are oxidized to methyl
ketones under the conditions of the iodoform
reaction. Secondary alcohols where the
hydroxyl group is bonded to the second carbon
atom from a terminal chain will be oxidized to
methyl ketones. 2-pentanol is an example
of this knid of alcohol (see below). The
methyl ketone that results form this oxidation
can then proceed into the iodoform reaction and
will result in giving a positive result for this
test.
 |
Permaganate
(KMnO4) Test
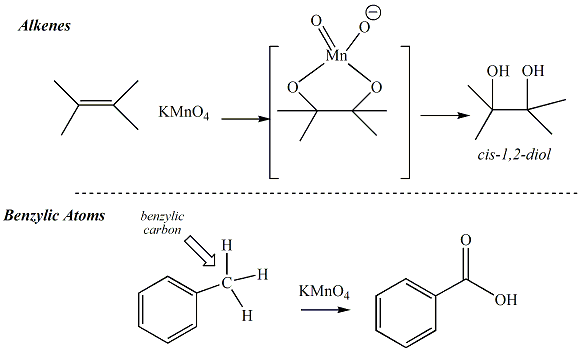
The permaganante test is generally used to test for alkene or alkyne functinal groups. Reaction of potassium permanganate with alkenes or alkynes occurs through an oxidation process. The permanganate oxygen atoms add to the pi system of the alkene or alkyne to generate a cyclic intermedicate that decomposes to a diol product. The diol product generated from alkynes reacts further to provide a a-hydroxcarbonyl compound as the final product. The purple permanganate reagent is reduced during the course of the oxidation reaction to give a colorless, yellow or brown solution. The transformation of the reaction mixture from a deep purple color to a yellow or brown solution indicates a positive result for this test. The pi bonds of an aromatic ring are not susceptible to oxidation by the permanganate reagent. However, permanganate may react with some benzylic atoms (atoms bonded to an sp2 hybridized C of a benzene ring) through a somewhat different reaction mechanism. The benzylic atom of toluene (for example) will be oxidized to a carboxylic acid. A postive test result will also frequently be observed when phenols are subjected to the permanganate reaction. |
|
 |
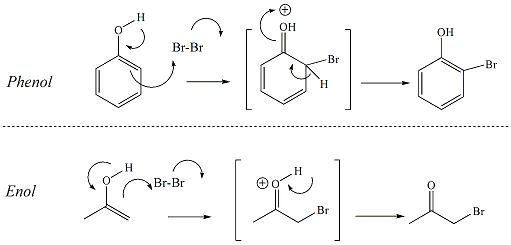
| Bromine/Water (Br2/H2O)Test The bromine water test
is generally used to identify alkenes, alkynes and
phenols. The bromine in water reagent reacts
with sites of unsaturation, even aromatic rings,
through a complex addition reaction.
For alkenes and alkynes, the reaction occurs
through a simple electrophilic addition. The pi
bond of enols will also react with bromine. But
for pi bonds of an aromatic ring, a hydroxyl group
must be bonded to the ring to stimulate the reaction
(i.e., a phenol). The hydroxyl group of the
phenol activates the ring toward reaction with the
electrophilic bromine. Bromine is a dark brown color but when
it reacts and becomes incorported into the product
via the addition react, the color dissipates and the
reaction mixture becomes yellow or sometimes
colorless. The dissipation of the brown color
of the bromine reagent indicates that the tested
compound contains an unsaturated hydrocarbon (alkene
or alkyne), phenol, or enol. The
advantage to using water as the solvent in this
reaction is that the polarity significantly enhances
the stability of the reaction intermediate and
increases the overall rate of the reaction. Additional catalysts are not
necessary. Aromatic
compounds without the phenol functional group rarely
react with bromine through this mechanism.
|
|
 |
Lucas Test The Lucas test is used
to identify compounds that contain alcohol functional
groups. The Lucas reagent is an aqueous
solution of strong acid (HCl) and zinc chloride. The alcohol starting
material must be sufficiently soluble in aqueous
environments (i.e., the Lucas reagent) for the
reaction to take place. Therefore,
only water-soluble, allylic (primary, secondary and
tertiary), tertiary
alkyl and very
few secondary
alcohols of low molecular weight will provide
positive results in this test. The reaction
that occurs in the Lucas test is an SN1
nucleophilic substitution. Only
alcohols that can generate stable carbocation
intermediates will undergo the reaction. The acid catalyst
activates the OH group of the alcohol by protonating
the oxygen atom. The
C-OH2+ bond breaks to generate
the carbocation, which in turn reacts with the
chloride ion (nucleophile) to generate an alkyl
halide product. An insoluble layer,
cloudiness, a change in color (red or orange) or
an emulsion will form with 1°, 2° or 3° allylic, 3° alkyl and some 2 ° alkyl alcohols. The
insoluble layer is the organic halide that forms
as the product in this SN1 reaction and this
organic halide (no hydrogen bonding) is not water
soluble, even if the starting alcohol (has
hydrogen bonding) is water soluble.
|
|
 |