Organic
Chemistry II | Lecture
| Laboratory
Organic Chemistry Laboratory II
Preparation
of Triphenylmethanol (Grignard Reaction)
Background Reading
Generation of Grignard Reagents
The Grignard reaction, named after its inventor, Nobel-prize-winning
chemist, Victor Grignard (1871-1935) is an extremely useful reaction
that
allows carbon/carbon bonds to be formed using an organometallic
intermediate.
These intermediates, commonly known as Grignard reagents, can be formed
by reacting alkyl, alkenyl or aryl halides with magnesium metal. The
organometallic
complex is characterized by the carbon-metal bond, such as that of
phenylmagnesium
bromide (Figure 1). Conversion of the alkyl or aryl halide to the
Grignard reagent results in an inversion of polarity associated with
the
carbon atom. The carbon atom of the alkyl/aryl halide has partial
positive character, while the same carbon develops partial negative
character
in the Grignard reagent. The reaction is typically carried out in
dry, aprotic solvents like diethylether or THF (tetrahydrofuran).
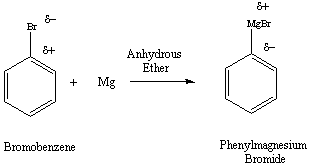
Figure 1: Formation of the Grignard Reagent from an Aryl
Halide
The Grignard reagent is a strong base and a strong nucleophile.
Specifically,
the carbon atom, with its negative character reacts with electrophilic
speciies. In the presence of acidic protons, the Grignard reagent will
break its organometallic bond and regenerate a hydrocarbon. Water and
alcohols,
for example, will have this effect (Figure 2). For this reason,
it
is very important that reactions using a Grignard reagent must take
place
under very dry conditions.
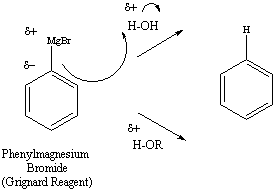
Figure 2: Reaction of the Grignard Reagent with Protons
Derived from Water or Alcohols
Once a Grignard reagent has been prepared, it is a very versatile
material.
It’s strongly nucleophilic character will cause interaction with any
electrophilic
carbon. Reactants with a carbonyl-containing functional groups, alkyl
halides
or alcohols (i.e.electrophilic proton) are a few examples of functional
groups that will react with a Grignard reagent.
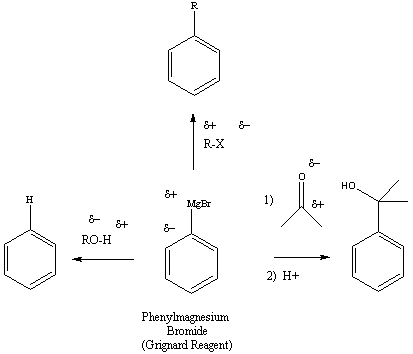
Figure 3: Reactions of the Grignard Reagent with Other
Functional Groups
Mechanism of the Reactions Involving Grignard Reagents:
Nucleophilic Acyl Addition and Nucleophilic Acyl Substitution
The mechanism of the reaction between the Grignard reagent and the
alkyl halide is completely analogous to the reaction of the Grignard
reagent
with a proton from water or an alcohol. The negative carbon of
the
Grignard reagent reacts with the electrophilic carbon of the alkyl
halide,
forming a new bond between the Grignard carbon and the alkyl halide
carbon.
The mechanism of the reaction between the Grignard reagent and a
carbonyl-containing
functional group is more complex. The negative carbon of
the
Grignard reagent attacks the carbonyl carbon, breaking the C-O pi bond
of the carbonyl-containing functional group to form a tetrahedral
intermediate.
A new C-C bond forms between the Grignard carbon and the carbonyl
carbon,
and the oxygen atom takes on a negative charge. If the starting
carbonyl-containig
functional group is an aldehyde or ketone, the negatively charged
oxygen reacts with a proton to generate an alcohol product (This
reaction
is referred to as a nucleophilic acyl addition)(Figure 4).
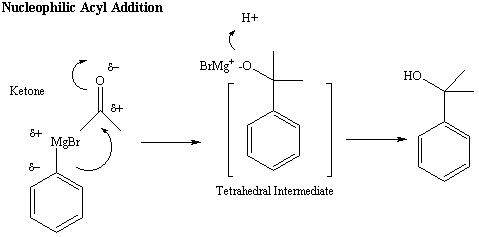
Figure 4: Mechanism of the Reaction of the Grignard
Reagent
with a Ketone
Nucleophilic Acyl Addition
If the starting carbonyl-containing functional group is a ester,
amide
or anhydride, the tetrahedral intermediate follows an alternative
path.
The electrons on the negatively charged oxygen are used to reform the
carbonyl
pi bond, forcing the C-N or C-O bond of the amide, ester or anhydride
to
break off as a leaving group, generating a ketone intermediate.
Because
ketones and aldehydes are more reactive in with the Grignard reagent
(The
carbonyl carbons of ketones and aldehydes are more electrophilic), the
ketone intermediate continues to react with a second equivalent of the
Grignard reagent to give a more highly substituted alcohol final
product.
This reaction is referred to as a nucleophilic acyl substitution.
(Figure
5)
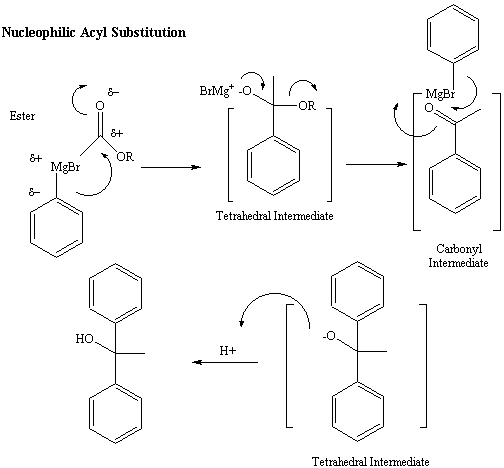
Figure 5: Reaction Mechanism for reaction of the Grignard
reagent
with an Ester
Nucleophilic Acyl Substitution
Carbon dioxide is a unique carbonyl-containing electrophilic
reagent.
Its addition in a 1:1 ratio with phenylmagnesium bromide will yield a
MgBr
salt of benzoic acid. Protonation of the salt with hydrochloric acid
will
yield the desired benzoic acid product, and the water-soluble MgBrCl
salt
(Figure 6).
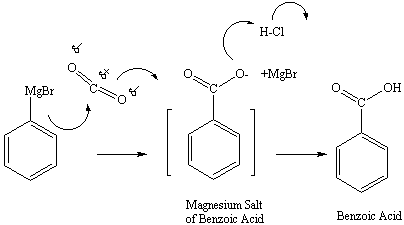
Figure 6: Reaction mechanism for Reaction of Grignard
reagent
with Carbon Dioxide






