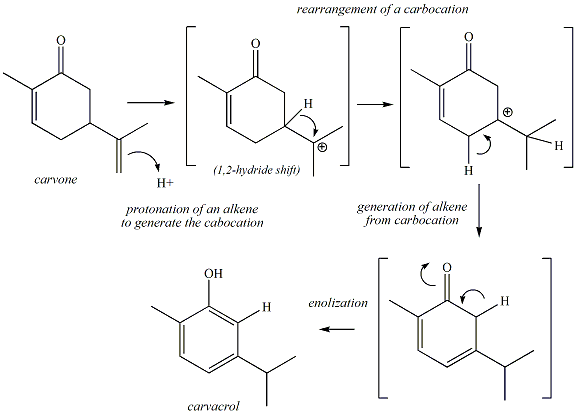
Carvone contains a ketone and two
alkene functional groups. One of the alkenes is conjugated with
the ketone (called a conjugated enone); the other alkene is not
conjugated. Treatment of carvone with strong acid promotes reaction of
the
non-conjugated alkene to
react with the acid to generate a carbocation, the first step of the
reaction illustrated in Figures 1 and 2. In this step, the pi
electrons of the alkene react with a proton. A new C-H bond is
generated between one of the carbons of the alkene
(C
2 in the Figure 2 below) and the proton, leaving behind a
carbocation on the other carbon atom
of the alkene (C
1 in Figure 2). The reaction occurs with the
regiochemistry shown to give
the more stable tertiary carbocation (rather than the less stable
primary carbocation), consistent with
Markovnikov
additon observed in electrophilic
addition reactions
.
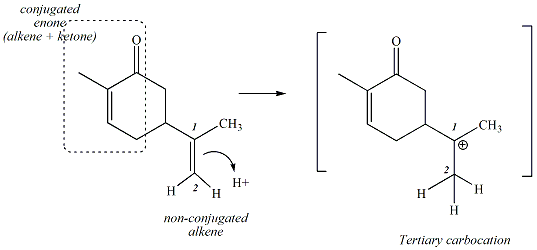 Figure 2:
Figure 2: Step One of the
Reaction: Protonation of the Non-Conjugated Alkene of Carvone
The tertiary carbocation that is generated in step one continues
to react by undergoing a
rearrangement.
This rearrangement involves migration of a hydrogen
atom from the C
1 carbon
(depicted in Figure 3 below) to the C
2 carbon. This
migration of a hydrogen atom is called a
1,2 -hydride shift, referring to
the shift in bonding of a H from one carbon (C
1) to an
adjacent carbon (C
2). The hydrogen atom takes
electrons from the C
1-H bond and uses those electrons to
form a new bond with the C
2 carbon. The net result is
that a new carbocation is generated on C
1. Carbocation
rearrangements generally occur only if the resulting carbocation is
equally stable or more stable that the original carbocation. The
new carbocation generated in this step of the reaction is a tertiary
carbocation, equivalent in stability to the original carbocation.
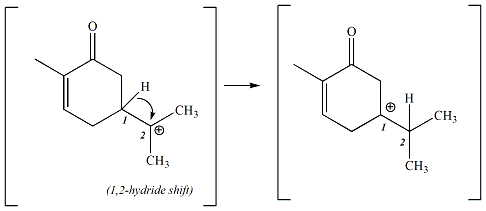 Figure 3:
Figure 3: Step 2 of the
Reaction: Carbocation Rearrangement (1,2-hydride shift)
The rearranged carbocation generated in step 3 continues to
react to generate a
conjugated diene.
In this step of the reaction, electrons from a C-H bond (C
1
in Figure 4), adjacent to the carbocation (C
2) are used to
form a new pi bond. The H atom of this bond loses its electrons
and becomes a proton (H+), thus regenerating the acid used in step one
and making the overall reaction catalytic (See section below on
catalyzed reactions). A new alkene is generated in this step of
the reaction that is conjugated with the aleken of the enone functional
group. The conigated diene is significantly more stable that the
tertiary carbocation.
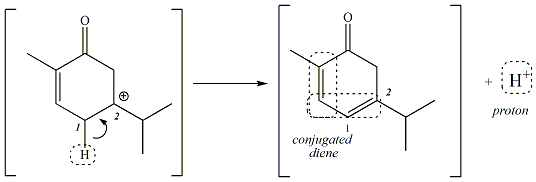
Figure 4: Step 3
of the Reaction: Formation of the Conjugated Diene
Step 4 of the Reaction:
Generation of Carvacrol by Enolization
The conjugated diene undergoes one final step in the
reaction to generate carvacrol. In this last step, the ketone is converted to a
phenol in a process referred to as enolization. Enolization is initiated
when one of the lone pairs on the oxygen of the carbonyl group accepts
a proton to generate an oxonium ion
(a positively charged oxygen atom). The electrons of the alpha
C-H bond shift over to become a new pi bond between the alpha carbon (C1
in Figure 5 below) and the carbonyl carbon (C2), and the pi
electrons of the carbonyl group become a lone pair on the oxygen,
removing the formal charge on oxygen and generating the phenol.
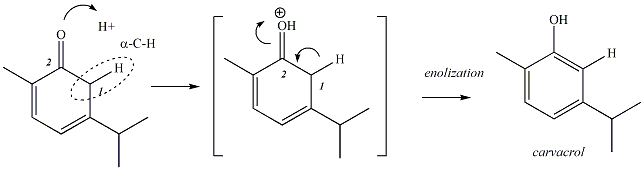
Figure 5:
Step 4 of the Reaction: Enolization to Form Carvacrol
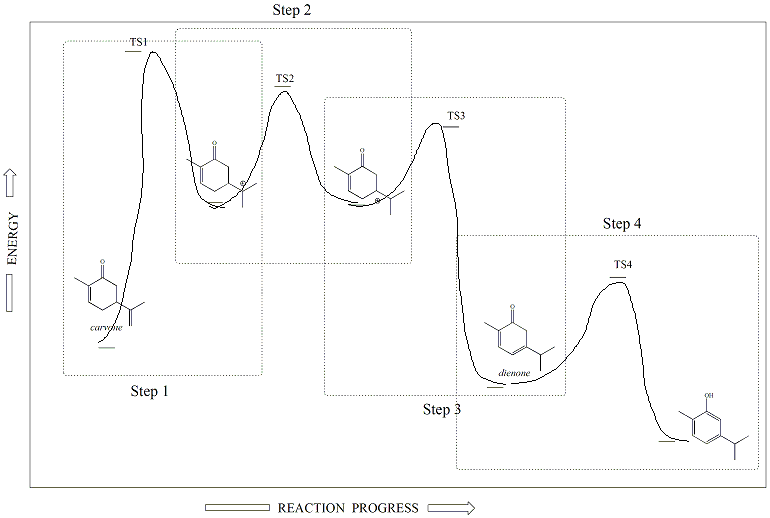
Thermodynamics and Kinetics of the Reaction
Generation
of carvacrol from carvone under acid-catalysis is a
thermodynamically-favorable reaction. The product (carvacrol) is
more stable than the starting material (carvone). Carvone has a
conjugated pi system and its stability is enhanced through
resonance, however the aromatic ring of carvacrol imparts even more
stablization to the product. The rate-determining step of the
reaction (slow step) is the first step that involves formation of the
carbocation from the unconjugated alkene of carvone.
Rearrangement of this carbocation to a second, tertiary carbocation
occurs in the next, fast step, followed by loss of a hydrogen to form a
stable, conjugated dienone. The dienone then enolizes in the
forth step to give the aromatic product, carvacrol. The
thermodynamics and kinetics of the reaction are illustrated in the
reaction energy diagram for this process, shown in Figure 6.
Figure 6: Reaction Energy Diagran for the Acid-Catalyzed
Conversion of Carvione to Carvacrol
The isomerization of carvone to carvacrol is an acid catalyzed
reaction. A catalyst is a special kind of reagent that is used to
initiate a reaction
by lowering the activation energy of the rate determining step of the
reaction. The catalyst is not a permanent reagent in the reaction
,
but rather is used temporarily and is eventually regenerated in the
reaction. The acid catalyst in this react is
used in step 1 of the reaction, the rate-determining step. The acid is
then regenerated in step 3 of the reaction when
the conjugated diene is formed.
Characterization
of the Reaction Product by Spectroscopic Analysis
The reaction of carvone with sulfuric acid to form carvacrol may be
monitored by
TLC. The phenol functional group of the carvacrol impacts
H-bonding characteristics in the product that are not present in the
starting material and thus the product would be expected to have
a lower Rf value when analyzed by TLC on silica gel. Comparison
of carvone with the reaction mixture allows for qualitative
determination of the reaction progress. Both the starting carvone
and the carvacrol product can be detected easily on a TLC plate by both
iodine and UV light, since both contain conjugated systems.
IR
spectroscopic analysis of the reaction mixture and the starting carvone
allows for assessment of the reaction . The reactant carvone
contains a conjugated ketone which will have a characteristic peak in
its IR spectrum in the range of 1650-1720cm-1. Conversely, the IR
spectrum of the carvacrol will lack the carbonyl peak but will contain
peaks consistent with the phenolic OH and the aromatic ring.
The proton NMR spectra of carvone and carvacrol will also allow the
product of this reaction to be distinghuished from the starting
material. The proton NMR spectrum of carvacrol should contain
characteristic peaks in the aromatic region of the spectrum that
correspond to the three aromatic protons. Two of the aromatic
protons should appear as doublets and the third proton should appear in
this region of the spectrum as a singlet. The benzylic methyl
will appear as a singlet in the region between 1.5-2.5 ppm in the
spectrum, and the isporopyl group should give rise to a doublet
(0-1.5ppm) and a multiplet ( 1.5-2.5ppm). The spectrum of
carvone will contain vinylic protons but no aromatic resonances.